Are you desperately looking for 'example case report form clinical research'? You will find your answers right here.
A case report grade (CRF) is organized to collect the patient data fashionable a clinical trial; its development represents a significant partially of the medical institution trial and bum affect study success.[1] Site personnel gaining control the subject's information on the CRF, which is amassed during their engagement in a medical institution trial.Author: Shantala Bellary, Binny Krishnankutty, One thousand S LathaCited by: Publish Year: 2014
Table of contents
- Example case report form clinical research in 2021
- Clinical research form templates
- Case report form in clinical trial slideshare
- Case report form fda
- Case report form pdf
- Case report forms crf
- Sample crf pdf
- Ich guidelines for case report form
Example case report form clinical research in 2021
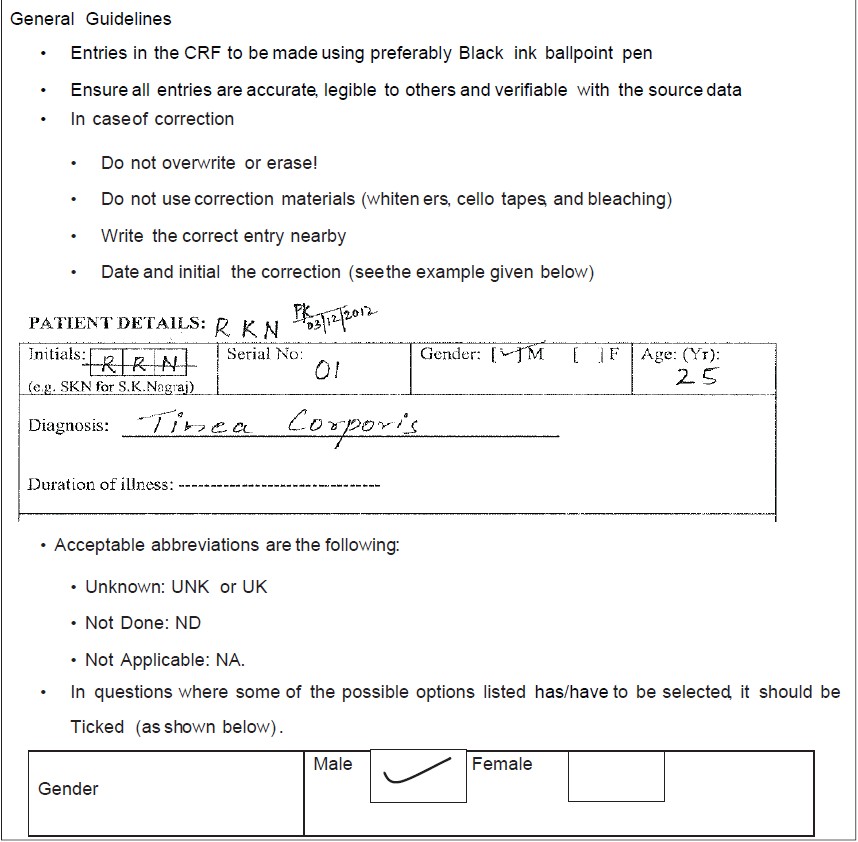 This picture shows example case report form clinical research.
This picture shows example case report form clinical research.
Clinical research form templates
 This picture representes Clinical research form templates.
This picture representes Clinical research form templates.
Case report form in clinical trial slideshare
 This picture representes Case report form in clinical trial slideshare.
This picture representes Case report form in clinical trial slideshare.
Case report form fda
 This picture shows Case report form fda.
This picture shows Case report form fda.
Case report form pdf
 This picture demonstrates Case report form pdf.
This picture demonstrates Case report form pdf.
Case report forms crf
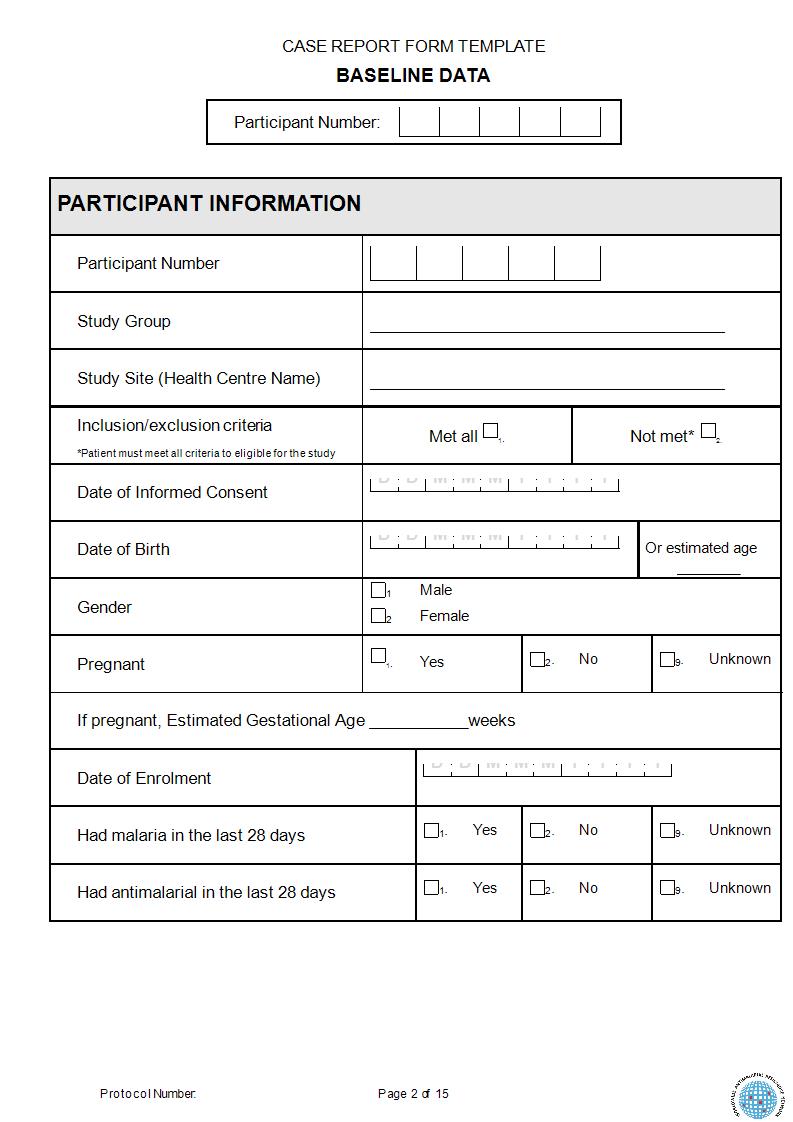 This picture illustrates Case report forms crf.
This picture illustrates Case report forms crf.
Sample crf pdf
 This image demonstrates Sample crf pdf.
This image demonstrates Sample crf pdf.
Ich guidelines for case report form
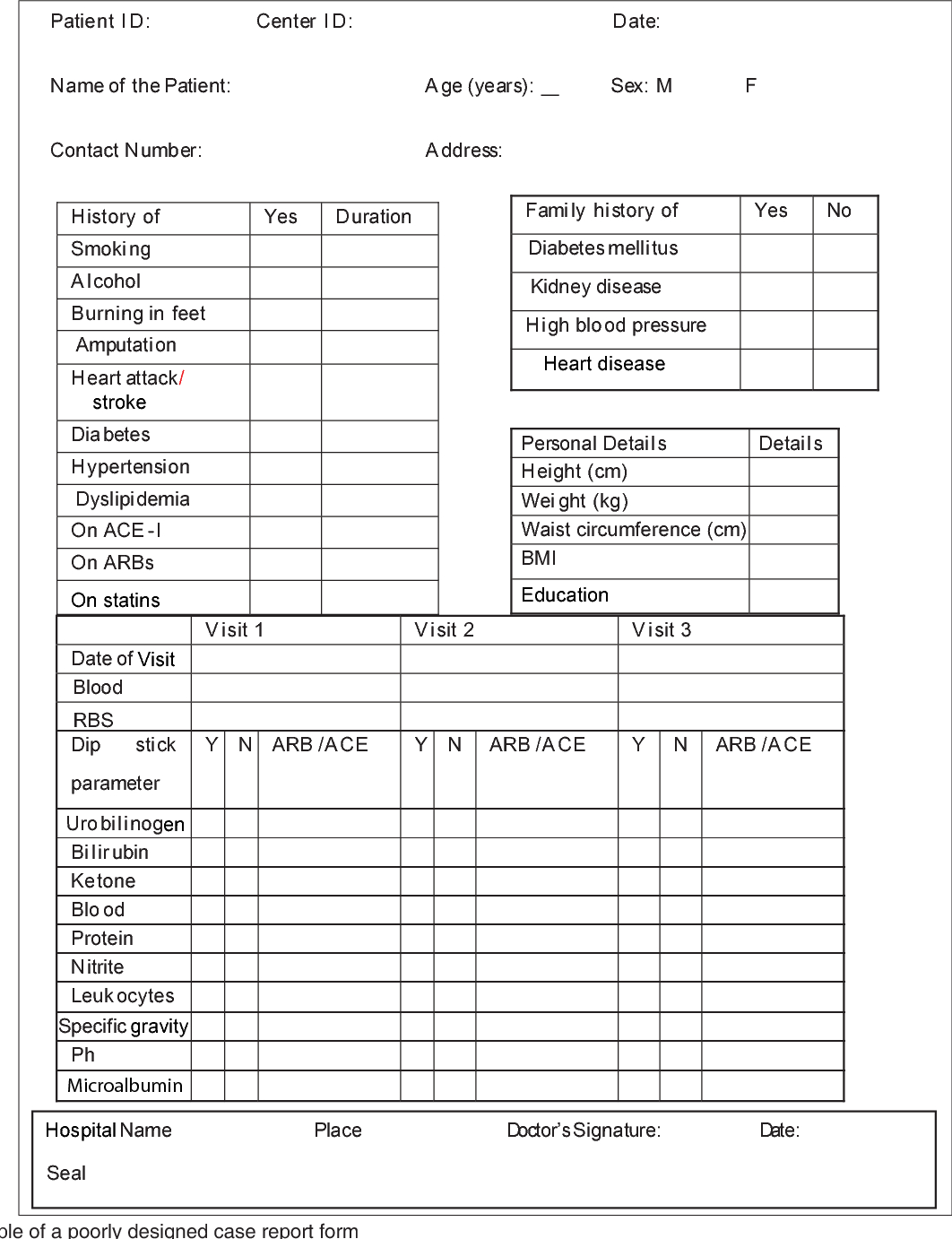 This picture shows Ich guidelines for case report form.
This picture shows Ich guidelines for case report form.
How are case report forms used in clinical research?
Case report forms (CRFs) are used to collect data in clinical research. Case report form development represents a significant part of the clinical trial process and can affect study success. Libraries of CRFs can preserve the organizational knowledge and expertise invested in CRF development and expedite the sharing of such knowledge.
What should be included in an abstract case report?
Abstract Case report form (CRF) is a specialized document in clinical research. It should be study protocol driven, robust in content and have material to collect the study specific data.
Where can I find case report form templates?
Case Report Form Templates. Case Report Form (CRF)/Source Document templates were created for University of Wisconsin-Madison researchers. These templates are consistent with the FDA’s CDASH (Clinical Data Acquisition Standards Harmonization) standards. The CDASH standards identify those elements that should be captured on a Case Report Form (CRF).
What are case report form templates for UW-Madison?
Case Report Form (CRF)/Source Document templates were created for University of Wisconsin-Madison researchers. The forms serve only as templates, and must be edited to meet the study data collection needs as described in the protocol.
Last Update: Oct 2021