Do you desperately look for 'write a balanced equation for the complete combustion of decane'? You will find all of the details here.
The balanced chemical equality for the rank combustion of decane is: 2 100 10 H 22 + 31 O 2 → 20 CO 2 + 22 H 2 O + Oestrus Energy ( Enthalpy) The hydrocarbon burning reaction releases oestrus energy and is an example of an exothermic chemical reaction. The reaction too has a counter enthalpy change (ΔH) value.Chemical formula: 100 10 H 22Energy density: MJ/kgMelting Point: 30 o CMolar mass: grams / mole
Table of contents
- Write a balanced equation for the complete combustion of decane in 2021
- C10h22+o2 co2+h2o
- Incomplete combustion of decane
- Write the balanced chemical equation for the complete combustion of heptane
- C10h22 cracking equation
- C10h22+o2=co2+h2o balanced equation
- C10h22 o2 = co2 + h2o reaction type
- C10h22
Write a balanced equation for the complete combustion of decane in 2021
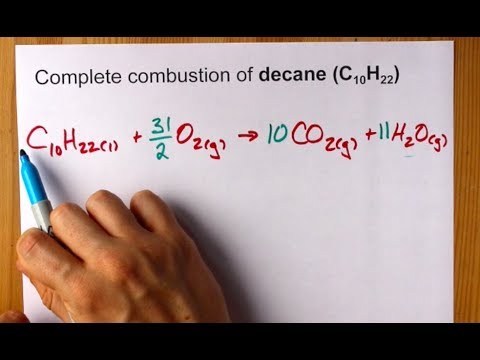 This picture illustrates write a balanced equation for the complete combustion of decane.
This picture illustrates write a balanced equation for the complete combustion of decane.
C10h22+o2 co2+h2o
 This picture demonstrates C10h22+o2 co2+h2o.
This picture demonstrates C10h22+o2 co2+h2o.
Incomplete combustion of decane
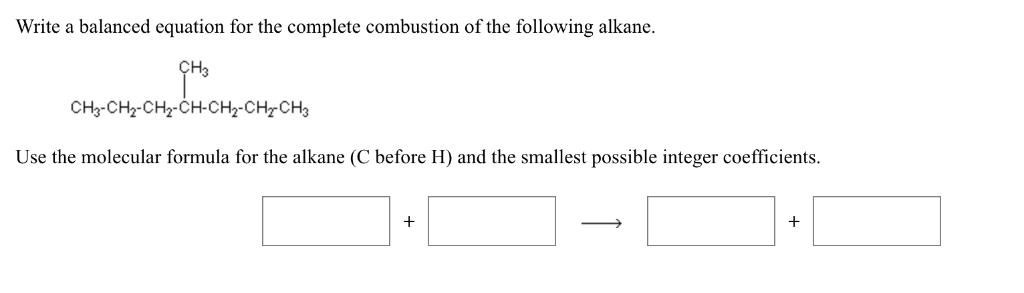 This picture demonstrates Incomplete combustion of decane.
This picture demonstrates Incomplete combustion of decane.
Write the balanced chemical equation for the complete combustion of heptane
 This image demonstrates Write the balanced chemical equation for the complete combustion of heptane.
This image demonstrates Write the balanced chemical equation for the complete combustion of heptane.
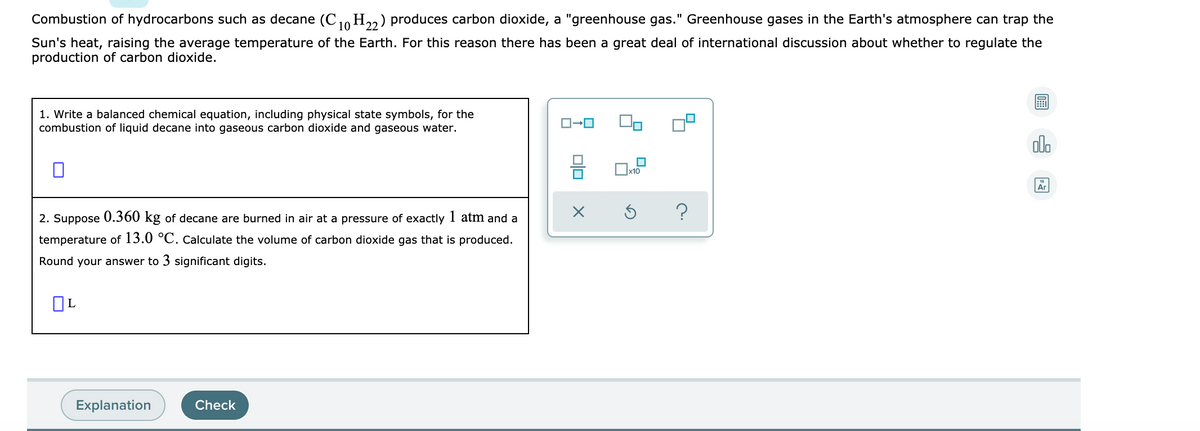
C10h22 cracking equation
 This picture demonstrates C10h22 cracking equation.
This picture demonstrates C10h22 cracking equation.
C10h22+o2=co2+h2o balanced equation
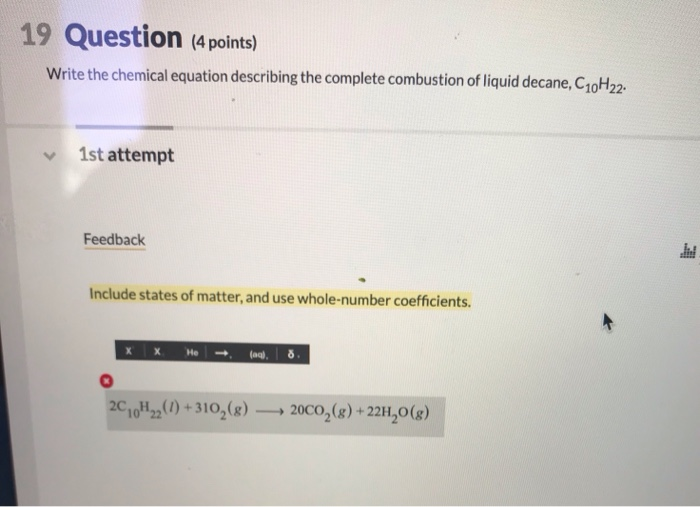 This image shows C10h22+o2=co2+h2o balanced equation.
This image shows C10h22+o2=co2+h2o balanced equation.
C10h22 o2 = co2 + h2o reaction type
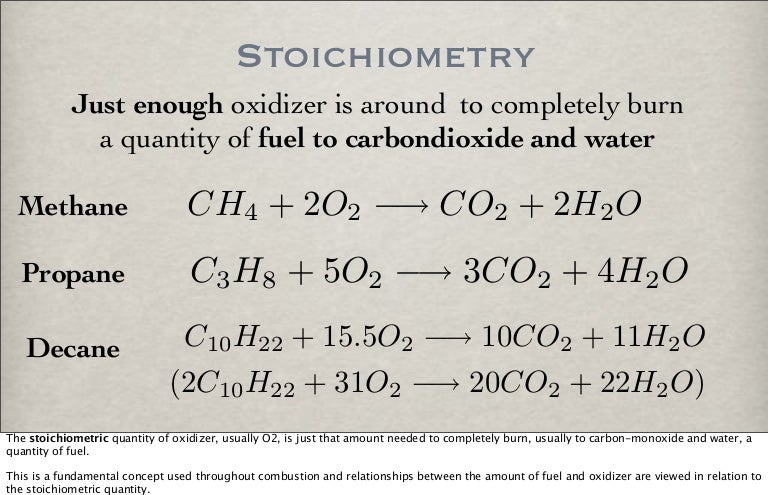 This picture shows C10h22 o2 = co2 + h2o reaction type.
This picture shows C10h22 o2 = co2 + h2o reaction type.
C10h22
 This image representes C10h22.
This image representes C10h22.
How to write and balance equation for decane?
Write and balance equation for the complete combustion of decane, C_ {10}H_ {22}. Write and balance equation for the complete combustion of decane, C10H22 C 10 H 22 . The complete combustion of decane under sufficient supply of oxygen gives carbon dioxide and water.
What is the balanced chemical equation for the complete combustion of decane?
The balanced chemical equation for the complete combustion of decane is: The hydrocarbon combustion reaction releases heat energy and is an example of an exothermic reaction. The reaction also has a negative enthalpy change (ΔH) value.
What is the equation for incomplete combustion of methane?
The word equation for the incomplete combustion of methane is: Methane + Oxygen > Carbon monoxide + Water What is the chemical equation for decane combustion? The chemical equation is:2 C10H22 + 31 O2 = 20) CO2 + 22 H2O What is the equation for incomplete combustion?
What happens when decane is used as a fuel?
Below is a table of some of the basic properties of decane. Like all hydrocarbons, decane undergoes hydrocarbon combustion when used as a fuel. The balanced chemical equation for the complete combustion of decane is: The hydrocarbon combustion reaction releases heat energy and is an example of an exothermic reaction.
Last Update: Oct 2021